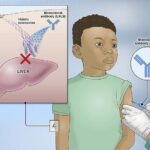
The academic health system is also the third hospital in the nation to treat a patient with Hemgenix, an FDA-approved gene therapy infusion for hemophilia.
Tampa, FL – January 19, 2024 – – Tampa General Hospital (TGH) announced that the TGH Cancer Institute recently completed Florida’s first successful infusion of Hemgenix, a gene therapy option for patients with severe hemophilia B — a rare bleeding disorder characterized by failure of the blood to clot properly. Under the direction of Dr. Nathan Visweshwar, an associate professor in the Division of Hematology and Medical Oncology in the USF Health Morsani College of Medicine, and a hematology and oncology specialist for Tampa General, the academic health system became the first in the state and the third hospital in the United States to administer this therapy with positive results.
“The infusion of this therapy is a hallmark of the TGH Cancer Institute’s dedication to propelling innovation to improve outcomes and quality of life in a meaningful way for our hematology/oncology patients,” said Dr. Eduardo M. Sotomayor, vice president and executive director of the TGH Cancer Institute. “We are proud that in offering this cutting-edge genetic therapy in our newly built, state-of-the-art infusion centers, we are uniquely positioned to address unmet needs for patients living with hemophilia B in our community and beyond.”
Hemgenix (etranacogene dezaparvovec) uses a gene therapy approach called gene transfer that is administered through intravenous (IV) infusion. This approach introduces a working, or functional, gene into liver cells to “instruct them” to produce factor IX protein (deficient in patients with hemophilia), which helps form blood clots to prevent prolonged or excessive bleeding.
“This is an exciting opportunity to pioneer this treatment option, which will allow us to continue expanding access to a wide range of therapies for patients with hemophilia in our community and throughout the state,” Visweshwar said.
Because the genes that cause hemophilia A and B are located on the sex-determining X chromosome, the disorder disproportionately affects males. According to the U.S. Centers for Disease Control and Prevention (CDC), as many as 33,000 males in the United States are living with hemophilia. Hemophilia B is a rarer form of the disorder, only prevalent in 3.7 cases per 100,000 U.S. males. The CDC reports that further studies are needed to assess the population of females living with hemophilia, both A and B.
“Our team’s successful administration of this gene therapy is the perfect case study for the distinct benefits an academic and research health system can deliver to patients,” said Dr. Abraham Schwarzberg, executive vice president, chief of Oncology, president of the Tampa General Provider Network (TGPN) and co-vice president of clinical and translational research. “The introduction of a new therapeutic option for this rare disease is a testament to our shared commitment to apply the latest research and clinical best practices to safely and effectively treat even the most complex conditions.”
This milestone is the latest achievement for the TGH Cancer Institute. U.S. News & World Report’s Best Hospitals list for 2023-2024 ranks the TGH Cancer Institute in the top 10% of hospitals in the nation for cancer care. Additionally, Newsweek named the Institute one of America’s Best Cancer Hospitals and one of the top 10 cancer facilities in Florida, and the academic health system was also recognized on a 2023 list of 100 Hospitals and Health Systems with Great Oncology Programs by Becker’s Hospital Review.