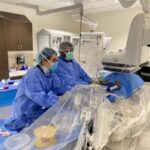
May 21, 2020 – Cleveland Clinic Florida has announced two new clinical trials to help patients who test positive for COVID-19. The clinical trials are in addition to the convalescent plasma trials, which began in late April.
The additional trials being conducted in Florida are:
A to Z Study: Vitamin C & Zinc
This study is a randomized outpatient clinical trial testing the use of Vitamin C and Zinc to determine whether they shorten the duration and type of symptoms, and prevent hospitalizations in patients with a new diagnosis of COVID-19. Participants will be given Vitamin C and Zinc treatment within 48 hours of a positive test and will be monitored for a two-week period.
Cleveland Clinic Florida will screen patients to determine if they meet criteria and are willing to enter the trial. Participants must be 18 or older. Exclusion criteria includes: pregnant and or breastfeeding women, patients with liver disease, end-stage renal disease, or history of kidney stones. Cleveland Clinic Florida’s goal is to enroll 520 participants in the study.
Canakinumab
This is an in-patient study specifically for admitted COVID-19 patients who have had a heart attack. COVID-19 has been known to cause heart attack, vascular inflammation and stroke in some patients. The study will determine whether early treatment with Canakinumab, an antibody therapy, will reduce the deterioration of cardiac function and prevent further damage, including progression to heart failure. The study also seeks to determine whether the drug prevents the onset of respiratory failure, need for ventilation and cardiac shock.
Enrollment for the trial will begin within the next two weeks.
“Cleveland Clinic Florida remains committed to the investigation of evidenced-based therapies to find innovative solutions that help patients diagnosed with COVID-19,” said Wael Barsoum, M.D., CEO and President of Cleveland Clinic Florida. “We are hopeful that these prospective treatments will help our team of experts better understand this disease and will ultimately provide the best care for patients moving forward.”
For nearly a month, Cleveland Clinic Florida has been conducting clinical trials for convalescent plasma therapy, which collects antibody-rich plasma from donors who have recovered from COVID-19 to use for patients currently struggling with the virus.
Cleveland Clinic Florida is working closely with its Ohio counterpart to lead the way in innovative research for COVID-19 patients. In April, Cleveland Clinic announced its Center for Global and Emerging Pathogens Research, and the soon-to-open Cleveland Clinic Florida Research and Innovations Center (FRIC) in Port St. Lucie, Florida, will be an essential part of research and treatment for COVID-19.
For more information about Cleveland Clinic Florida, please visit www.clevelandclinicflorida.org.
About the Cleveland Clinic Florida Region
The Cleveland Clinic Florida region is a nonprofit, multi-specialty healthcare provider that integrates clinical and hospital care with research and education. The Florida region now includes Cleveland Clinic Indian River Hospital, Cleveland Clinic Martin Health, and Cleveland Clinic Weston Hospital, with five hospitals and numerous outpatient centers in Broward, Palm Beach, Martin, St. Lucie and Indian River Counties. The Florida region is an integral part of Cleveland Clinic in Ohio, where providing outstanding patient care is based upon the principles of cooperation, compassion and innovation. Physicians at Cleveland Clinic are experts in the treatment of complex conditions that are difficult to diagnose. For more information about Cleveland Clinic Florida, visit www.clevelandclinicflorida.org. Follow us on Twitter and Facebook.